x
Example of a product with two different pack sizes. (next)
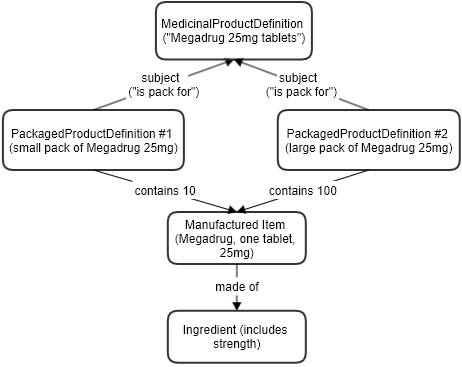
Product [documentation R4 R5]
id: megadrug
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0316e
- Name: Megadrug 25mg tablets
Package (1 of 2) [documentation R4 R5]
id: megadrug-pack-10
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e
Packaging
Amount: 10 tablet
Found a parent (PackagedProductDefinition/item, id: megadrug-pack-10 fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)
which is linked to this by resource.id
which is linked to this by resource.id
Found a parent (PackagedProductDefinition/item, id: megadrug-pack-100 fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)
which is linked to this by resource.id
Manufactured Item [documentation R4 R5]which is linked to this by resource.id
id: megadrug-tablet
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0319e
- powder for solution injection (http://example.org.uk/fhir/doseform)Status: active
Dose Form: powder for solution injection (http://example.org.uk/fhir/doseform)
Package (2 of 2) [documentation R4 R5]
id: megadrug-pack-100
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e
Packaging
Amount: 100 tablet
Found a parent (PackagedProductDefinition/item, id: megadrug-pack-10 fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)
which is linked to this by resource.id
which is linked to this by resource.id
Found a parent (PackagedProductDefinition/item, id: megadrug-pack-100 fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)
which is linked to this by resource.id
Manufactured Item [documentation R4 R5]which is linked to this by resource.id
id: megadrug-tablet
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0319e
- powder for solution injection (http://example.org.uk/fhir/doseform)Status: active
Dose Form: powder for solution injection (http://example.org.uk/fhir/doseform)
↸arrow indicates that this RegulatedAuthorization resource points back to the parent MedicinalProductDefinition, instead of being linked forwards from it - via RegulatedAuthorization.subject
Authorisation [documentation R4 R5]
Authorisation [documentation R4 R5]
id: megadrug-auth
fullUrl: urn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e
Description: Authorized by EMFDA on 1st Jan 2016
Warning - Found Ingredient that is not referenced (ingredient-mega-drugurn:uuid:e19deb42-7137-48b0-aef1-d37019d0310e)
Warning - Found RegulatedAuthorization with no type (megadrug-authurn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)
Warning - Found RegulatedAuthorization with no type (megadrug-authurn:uuid:e19deb42-7137-48b0-aef1-d37019d0317e)